With the advent of clean fuels and the constantly changing sources of crude, it’s important for refiners to understand the many variables for control and optimization to maximize profitability. Read on to discover what crude distillation is and why crude distillation is so important in the oil refining process.
Overview
Crude distillation is the first step in processing crude oil into its different end products, including gasoline, kerosene, diesel, jet fuel, asphalt, and more. Crude stills are the oldest means for processing oil. The purpose of crude distillation is to recover the light materials from the oil. This process begins with a fractional distillation column, which separates the various components of the crude oil by their size, weight, and boiling point.
The crude oil mixture is heated using high pressure steam. The vapors from this liquid rise up into the fractional distillation column, which contains several trays. Each tray is designated for a different kind of oil. The tray captures its designated vapor as it is separated from the other vapors according to their individual boiling points. So the substance with the lowest boiling point (car gasoline) will condense at the highest point in the column and the substance with the higher boiling point will condense lower down in the column.
Bottoms Up! – Typical Distillation Column
In a typical distillation column, the bottom serves as the reboiler to add heat. The temperature and pressure are highest here, where the light components are separated from the falling liquid. Above this, the trays are housed. The trays capture the rising vapors and the different liquids. Higher up, the feed separates the various liquids (heating oil, diesel, jet fuel, car fuel, etc). And the the very top of the column, the condenser removes the heat and the heavy components are stripped from the rising vapors. The temperature is the coolest up here and the pressure is the lowest.
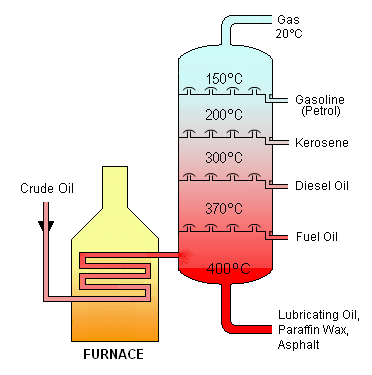
Photo Source: Users Psarianos, Theresa knott on en.wikipedia [CC BY-SA 3.0 (http://creativecommons.org/licenses/by-sa/3.0/)], via Wikimedia Commons https://commons.wikimedia.org/wiki/File:Crude_Oil_Distillation.png
After the trays collect their designated liquid fractions, they are then either routed to cooling condensers or directly to storage tanks where they undergo further chemical processing.
OMV, which produces and markets oil and gas as well as other petrochemical solutions, has produced an informative video on crude oil distillation.
Refining NZ also put out an interesting video that covers the basic overview of distillation (and they talk about whiskey!):
Chemical Process for Crude Distillation
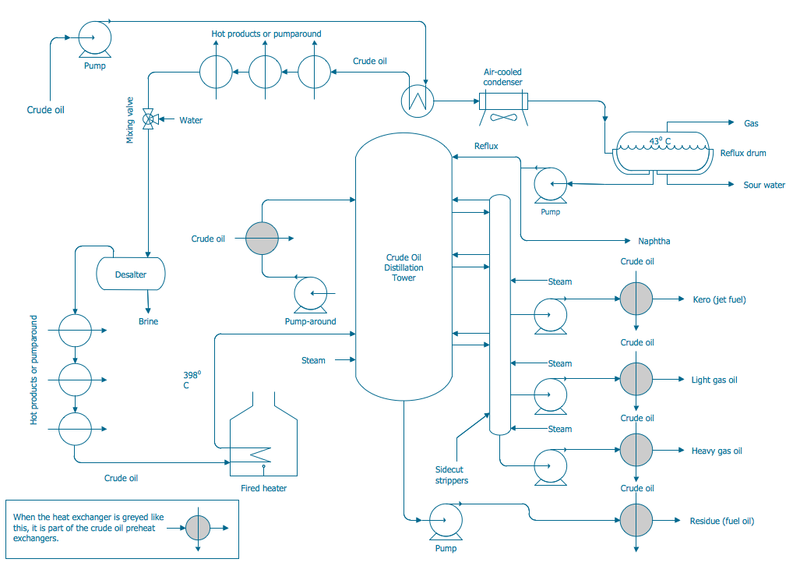
Photo Source: Wikimedia user Cmdrjameson. https://commons.wikimedia.org/wiki/File:Engineering-Chemical-Process-PFD-Crude-Oil-Distillation-Unit.pnghttp:/ File name: Engineering-Chemical-Process-PFD-Crude-Oil-Distillation-Unit.png
Why is crude important?
As the Planete Energies website explains, refining crude oil is important because without the refining process, we couldn’t use any of it. According to the website, “each crude oil is very different and made up of a large number of hydrocarbon molecules. The lightest molecules, dissolved gases, have 1 to 4 carbon atoms, while the heaviest have more than 20. These molecules are present in varying proportions depending on the deposit, meaning that each oil has its own composition and properties. Some crudes are black and viscous and contain a lot of heavy molecules; others are brown, more fluid and lighter. Each also contains a certain amount of dissolved gas and highly corrosive products, such as sulfur or acids, which can sometimes be toxic.”
As such, it is impossible to develop a universal operating system for all the uses of crude. This article offers more information on the three stages of refining, which include separate, conversion, and treating.
Crude Challenges
According to AMACS, a leading manufacturer of separation and phase containing process internals, there are several things that can go wrong with crude distillation. They include the use of a wrong fuel or catalyst, improper pressure balance, improper column mass balance, reflux ratio, and the regular analysis of the distillation column.
We will address these challenges and much more in a new crude distillation course offered at RefComm® Galveston 2019. Crude Unit Distillation, Troubleshooting, and Desalting will provide participants with the knowledge necessary to understand, troubleshoot and optimize crude oil distillation and desalting. The discussion will focus on design considerations, equipment, components, controls, operation, performance, and troubleshooting tactics. The course will include teamwork activities, analysis of case studies, and interactive workshops to fully understand the constant adjustments required to maintain steady operation, capacity and product quality, as well as leverage that information to maximize your refinery production and profitability.
This course is for engineers, technical and troubleshooting staff, maintenance and operations personnel as well as design, construction and service providers involved in or interested in the operation, monitoring and optimization of crude oil distillation and desalting.
You Will Learn
- Crude Unit – History Overview and Unit Configurations, World Benchmarking
- Crude Unit – Safety and Incident Overview
- Crude Oil Composition and Origin – Crude Assay Review
- Crude Oil Chemistry
- Other Crude Unit Feeds and potential issues
- Crude Fractionation and Column Designs – Core Fractionation Course
- Desalting and Desalter Issues and Troubleshooting
- Flash Vessel and Column – Foaming issues Antifoam and Foam Suppression equipment design
- Crude Preheat Sections, Operations, Monitoring and Issues
- Atmospheric Section Configurations, Operation, Issues, and Process/Operation Monitoring
- Vacuum Section Configurations, Operation, Issues, and Process/Operation Monitoring
- Crude Unit Process Monitoring Templates
- Major Crude Unit Incidents with Class Room Team Breakouts Troubleshooting – 4 hours
- Crude Heater Decoking – Pigging and Smart Pigging
- Crude Unit Start-Up
- Crude Unit Shutdown and Decontamination – Flareless
Appendix
- Crude Unit Safety Incident Presentations and Video
- Additional Reading and References
Visit our RefComm® page for more information and to register.
_______________
Follow our RefComm Showcase page on LinkedIn.
Follow us on Facebook.








I think your PFD is missing the gas off the top of the column that crosses with the cold feed before being condensed with the air cooled exchanger.
Richard thanks for your input!